Which of the Following Is Used to Determine Stoichiometric Factors
Convert units of a given substance to moles. The balanced chemical equation provided equivalences that we used to construct conversion factorsFor example observe the following balanced chemical equation.

Reaction Stoichiometry Boundless Chemistry
Your email address will not be published.
. Determine the stoichiometric factor in the following balanced chemical equation. 2H₂g O₂g 2H₂Og The mole ratio between O₂ and H₂O is 1 mol O₂2 mol H₂O. Another conversion factor that is commonly used in stoichiometry is the molar mass or gmol.
Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant or to yield a given amount of product and so forth. Leave a Reply Cancel reply. Here we make use of ratios from the balanced equation.
In Chapter 5 Stoichiometry and the Mole we related quantities of one substance to another in a chemical equation by performing calculations that used the balanced chemical equation. The ratio is used as a conversion factor. Chemical formulas provide the identities of the reactants and products involved in the.
Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant or to yield a given amount of product and so forth. The coefficients in the balanced equation are used to derive stoichiometric factors that permit computation of the desired quantity. Which of the following is used to determine stoichiometric factors.
One can calculate the empirical formula from the percent composition. In general all the reactions that take place are dependent on one main factor how much substance is present. Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products.
D 6 mol H 2 O4 mol NH 3. The balanced chemical equation for the reaction and either the masses of solid reactants and products or the volumes of solutions of reactants and products can be used to determine the amounts of other species as illustrated in the following examples. Coefficients in a balanced chemical equation coefficients in an unbalanced chemical equation total number of reactants in a chemical equation.
Perform stoichiometric calculations involving mass moles and solution molarity. 4NH 3 5O 2 4NO 6H 2 O. Required fields are marked.
Inverted molar mass In the following stoichiometry problem how many moles of oxygen are produced when 30 mol of KClO3 decompose completely. The coefficients in the balanced equation are used to derive stoichiometric factors that permit computation of the desired quantity. A stoichiometric factor gives a mathematical approach to looking at these types of problems.
A balanced chemical equation provides a great deal of information in a very succinct format. Which of the following stoichiometric factors would be used to convert from moles of NH 3 to moles of H 2 O for the reaction. Perform stoichiometric calculations involving mass moles and solution molarity.
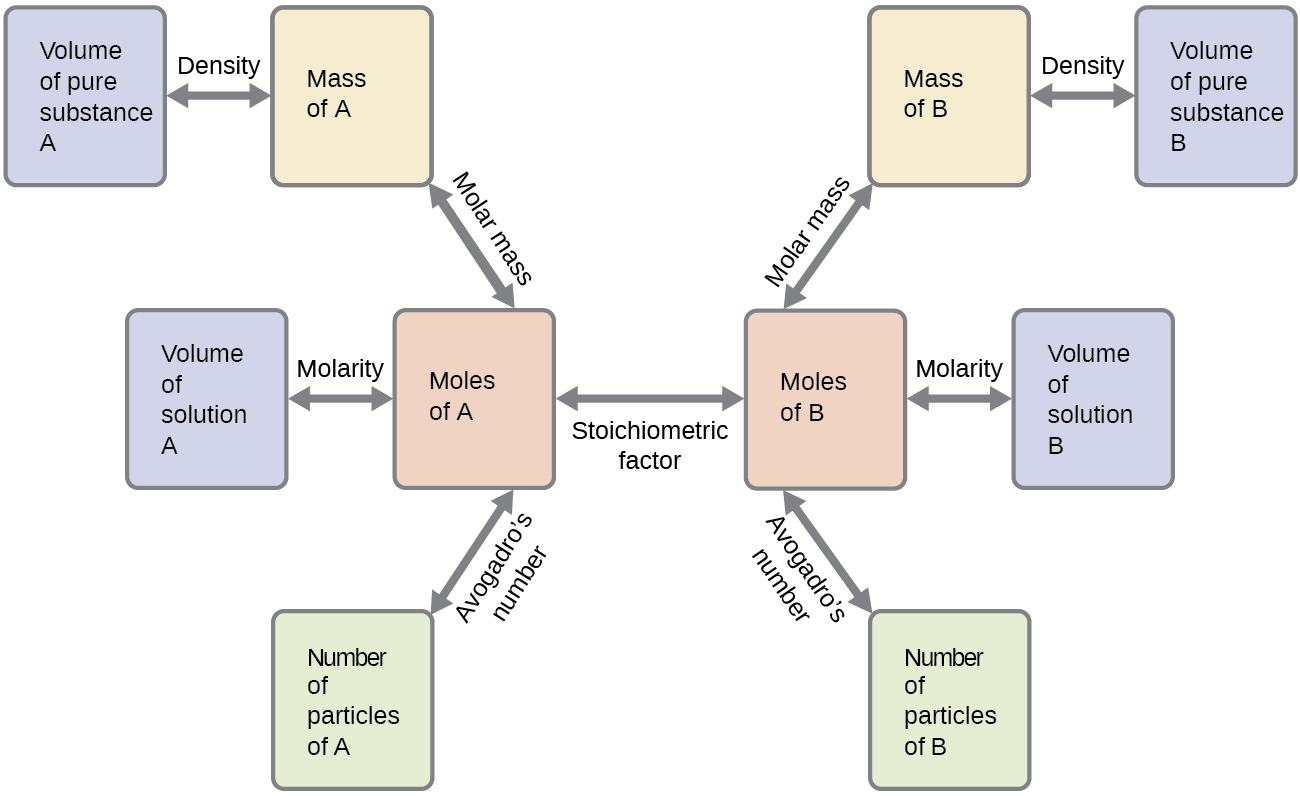
An Expanded Flowchart for Stoichiometric Calculations. Stoichiometry is an important concept in chemistry that helps us use balanced chemical equations to calculate amounts of reactants and products. Either the masses or the volumes of.
Select the correct answer below. Which of the following is used to determine stoichiometric factors. Chemical formulas provide the identities of the reactants and products involved in the.
A conversion factor is ALWAYS equal to 1. Mole ratios are used as conversion factors between products and reactants in stoichiometry calculations. Is a 11 rario correct.
Use balanced chemical equations to derive stoichiometric factors relating amounts of reactants and products. Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant or to yield a given amount of product and so forth. Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant or to yield a given amount of product.
Move mouse over terms in equation. Here are some examples of conversion factors. Determine the stoichiometric factor in the following balanced chemical equation.
The mole ratio between H₂ and H₂O is 2 mol H₂2 mol H₂O. Almost all stoichiometric problems can be solved in just four simple steps. The coefficients in the balanced equation are used to derive stoichiometric ratios that permit computation of the desired quantity.
The coefficients in the balanced equation are used to derive stoichiometric factors that permit computation of the desired quantity. To illustrate this idea consider the. The stoichiometric factor will always have the same form with the.
Applying Conversion Factors to Stoichiometry Now youre ready to use what you know about conversion factors to solve some stoichiometric problems in chemistry. The conversion factor used to convert from grams to moles is _____. Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant or to yield a given amount of product and so forth.
To illustrate this idea consider the. The coefficients in the balanced equation are used to derive stoichiometric factors that permit the computation of the desired. While the above example was relatively simple to do in your head it could have been written out mathematically.
To illustrate this idea consider the. The compound para-aminobenzoic acid you may have seen it listed as PABA on your bottle of sunscreen is composed of carbon 6131 hydrogen 514 nitrogen 1021 and oxygen 2333. A 5 mol O 2 4 mol NH 3.
A balanced chemical equation provides a great deal of information in a very succinct format. C 4 mol H 2 O6 mol NH 3. Balanced chemical equations are used in much the same fashion to determine the amount of one reactant required to react with a given amount of another reactant or to yield a given amount of product and so forth.
The easiest way to do stoichiometric calculations involves using conversion factors. The conversion factor that is always used in stoichiometry problems is the mole to mole ratio for elements or compounds in the balanced equation. Which of the following is used to determine stoichiometric factors.
The coefficients in the balanced equation are used to derive stoichiometric factors that permit computation of the desired quantity. For example in the reaction. Is a 11 rario correct.
A conversion factor is a ratio or fraction which represents the relationship between two different units. B 4 mol NH 3 6 mol H 2 O.
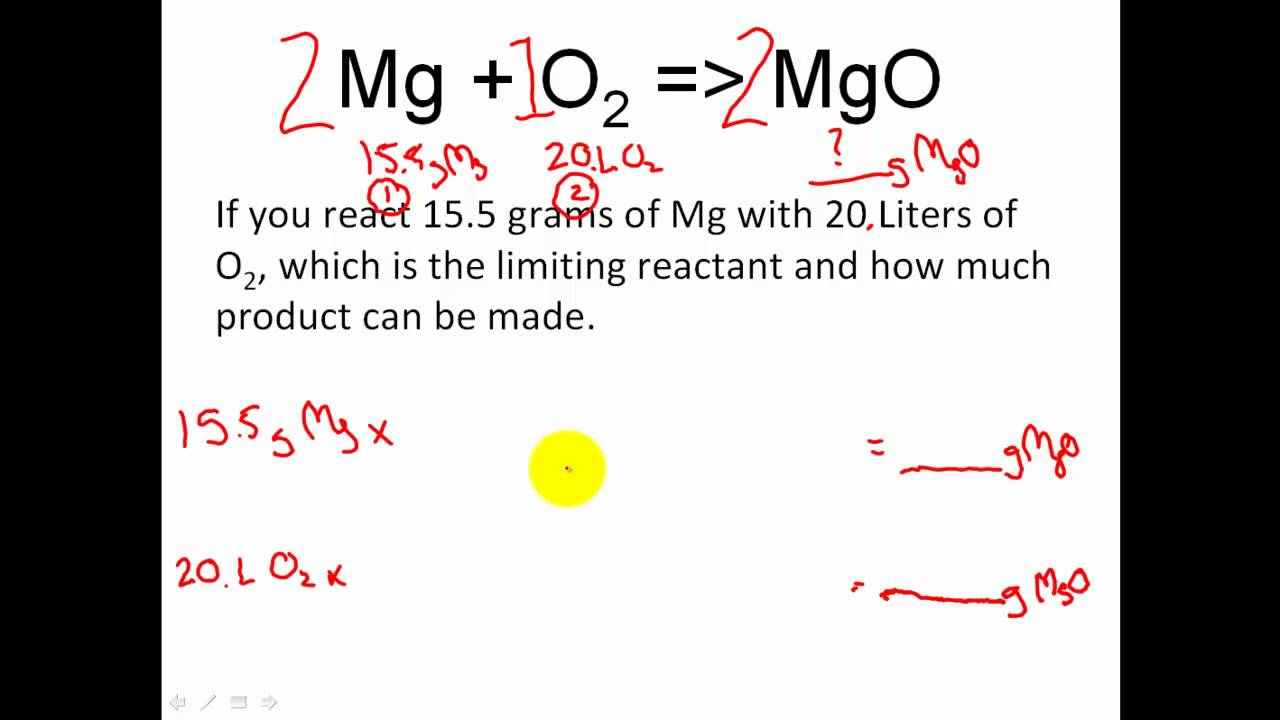
Reaction Stoichiometry Boundless Chemistry

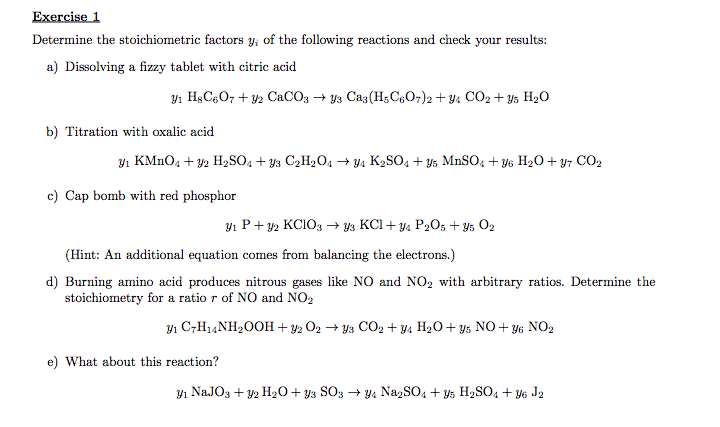
Exercise 1 Determine The Stoichiometric Factors Yi Of Chegg Com
No comments for "Which of the Following Is Used to Determine Stoichiometric Factors"
Post a Comment